Abstract
Introduction: Over the past decades, different medicinal products have been used for treatment of multiple myeloma in clinical studies, including new generations of proteasome inhibitors and immunomodulating agents, monoclonal antibodies, histone deacetylase inhibitors etc. The use of these treatment modalities results in improvement of overall survival (OS). However, taking into account high cost of these drugs, their availability in real clinical practice remains limited.
Aims: to determine OS in MM patients in real clinical practice in Russia.
Materials and methods: 2494 patients aged 24 to 90 years (median age=63 years) with newly diagnosed MM were enrolled into the prospective multicenter registration study from January 1, 2015 till July 1, 2017. The patients received treatment at 43 hematology clinics in Russia. The diagnosis was made based on the IMWG 2014 criteria. Most patients (78%) had stage III of the disease (Durie-Salmon) at the moment of the diagnosis, while stage II and stage I disease was diagnosed in 12% and 10% of cases, respectively. Kidney injury at the time of the disease onset was revealed in 22% of patients; renal replacement therapy (programmed hemodialysis) at the moment of the diagnosis was required in 9% of patients (GFR [Cockcroft-Gault Formula] <15 mL/min). Bone plasmacytomas detected by CT scan or MRI were observed in 25% of patients; extramedullary lesions were revealed in 5% of cases. Evaluation of antitumor response and survival analysis were carried out for 1042 patients enrolled in the registration trial in 2015. Bortezomib-based first-line therapy regimen was used in 92% of patients; 7% of patients received chemotherapeutic agents only; 1% of patients refused to receive any treatment. Autotransplantation was performed in 92 (16%) patients under 65 years of age at 12 centers for transplantation. In most cases (70%), the second-line therapy regimens included immunomodulating agents (lenalidomide); in 15% of cases, only chemotherapeutic agents were used for treatment of the disease recurrence; repeated treatment with bortezomib was used in 10% of cases; further generations of drugs (pomalidomide, carfilzomib) were prescribed in 5% of cases. Statistical survival analysis was conducted using the Kaplan-Meier estimate. Statistical software package SAS 9.1 was applied for calculations.
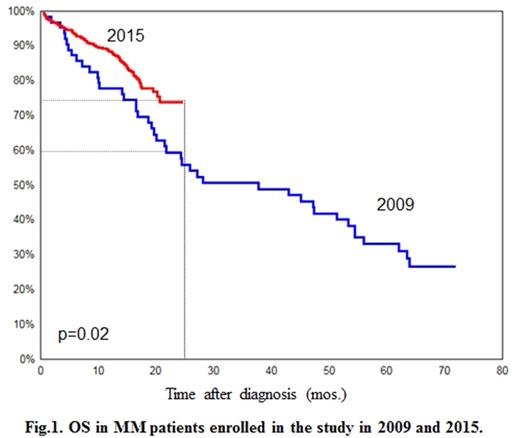
Results: overall anti-tumor response as a result of the induction therapy was achieved in 64% of patients. Two-year OS was 72%; median period of observation was 17 months. During the study period, 192 fatal outcomes were registered: disease progression - 55%; hemorrhagic complications - 21%; infections - 12%. In the course of the study, the values of OS in MM patients registered in 2015 were compared to the archive data of the registry - 170 patients registered in 2009-2011 who had received treatment at five hematology clinics in Russia. Two-year OS in patients registered in 2015 was statistically significantly higher (p=0.02; log-rank test) compared to patients registered in 2009: 72% versus 60% (Fig. 1).
Discussion: The results of this prospective study represent real clinical practice of therapy for MM in Russia. High frequency of newly diagnosed advanced stages of the disease was observed. The first-line therapy regimens in the vast majority of MM patients in Russia include bortezomib, the efficacy of which is comparable to that observed in other regions of the world. The results of the study have demonstrated significant improvement of OS in patients with MM diagnosed in 2015.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal